Computational study of polycyclic aromatic hydrocarbons growth by vinylacetylene addition

Computational study of polycyclic aromatic hydrocarbons growth by vinylacetylene addition
P. Liu, Y. Zhang, Z. Li, A. Bennett, H. Lin, S.M. Sarathy, W.L. Roberts
Combustion and Flame, 202, pp. 276-291, (2019)
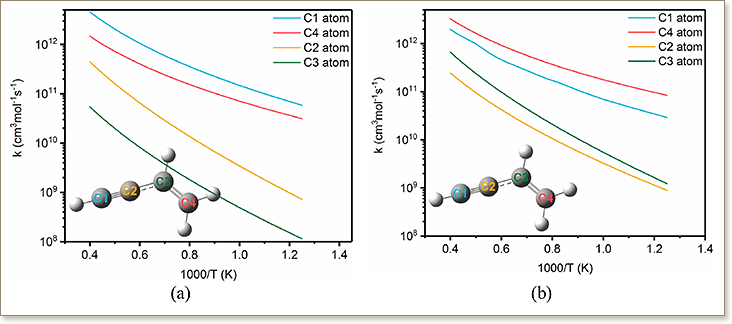
The growth of polycyclic aromatic hydrocarbons (PAH) can proceed via multiple chemical mechanisms. The mechanism of naphthyl radical and vinylacetylene (C4H4) addition reaction has been systematically investigated in this computational study. A combination of DFT/B3LYP/6-311+G(d,p), CCSD/6-311+G(d,p) and CBS-QB3 methods were performed to calculate the potential energy surfaces. It revealed that the products, including phenanthrene, anthracene, a PAH with a five-membered ring structure, and PAH with a C4H3 radical substitution, can be formed in A2-1 (1-naphthyl)+C4H4 and A2-2 (2-naphthyl) +C4H4 reaction networks. The reaction rate constants at 0.1-100 atm were evaluated by RRKM theory by solving the master equation in the temperature range of 800–2500 K, which showed that the rate constants of reactions A2-1 (A2-2)+C4H4→product+H are highly temperature-dependent but nearly pressure-independent. The distribution of products was investigated in a 0-D batch reactor, wherein the initial reactant concentrations were taken from experimental measurements. The results showed that adduct intermediates were the main products at low temperature (T < 1000 K), and the phenanthrene and PAH with C4H3 radical substitution became the dominant products at temperatures where PAHs and soot form in flames (T > 1000 K). It was observed that a significant amount of phenanthrene is formed from PAH with a C4H3 radical substitution with the assistance of H atom. Reaction pathway sensitivity analysis for the PAH radical+C4H4 reaction system was performed and showed that the new benzene rings are more likely to be generated near the zig-zag edge surface site instead of the free edge. For the development of a PAH mechanism, the analogous treatment of rate constants for larger PAH radical + C4H4reaction system are discussed. The formation rate of naphthalene from the reaction of phenyl+C4H4 was found to be very close to that of phenanthrene from the reaction of naphthyl+C4H4, suggesting that the analogous treatment of the rates is reasonable in PAH mechanisms.

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
© King Abdullah University of Science and Technology. All rights reserved