A comprehensive iso-octane combustion model with improved thermochemistry and chemical kinetics

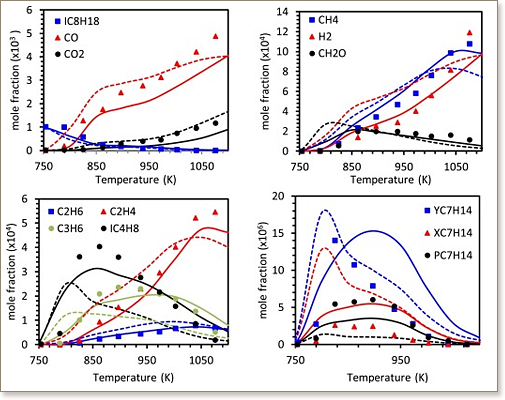
Iso-Octane (2,2,4-trimethylpentane) is a primary reference fuel and an important component of gasoline fuels. Moreover, it is a key component used in surrogates to study the ignition and burning characteristics of gasoline fuels. This paper presents an updated chemical kinetic model for iso-octane combustion. Specifically, the thermodynamic data and reaction kinetics of iso-octane have been re-assessed based on new thermodynamic group values and recently evaluated rate coefficients from the literature. The adopted rate coefficients were either experimentally measured or determined by analogy to theoretically calculated values. Furthermore, new alternative isomerization pathways for peroxy-alkyl hydroperoxide (ȮOQOOH) radicals were added to the reaction mechanism. The updated kinetic model was compared against new ignition delay data measured in rapid compression machines (RCM) and a high-pressure shock tube. These experiments were conducted at pressures of 20 and 40 atm, at equivalence ratios of 0.4 and 1.0, and at temperatures in the range of 632–1060 K. The updated model was further compared against shock tube ignition delay times, jet-stirred reactor oxidation speciation data, premixed laminar flame speeds, counterflow diffusion flame ignition, and shock tube pyrolysis speciation data available in the literature. Finally, the updated model was used to investigate the importance of alternative isomerization pathways in the low temperature oxidation of highly branched alkanes. When compared to available models in the literature, the present model represents the current state-of-the-art in fundamental thermochemistry and reaction kinetics of iso-octane; and thus provides the best prediction of wide ranging experimental data and fundamental insights into iso-octane combustion chemistry.

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
© King Abdullah University of Science and Technology. All rights reserved