Antiknock quality and ignition kinetics of 2-phenylethanol, a novel lignocellulosic octane booster

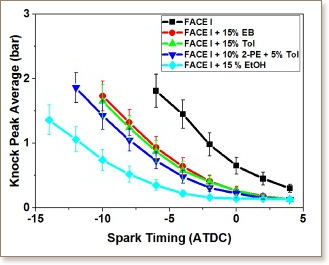
High-octane quality fuels are important for increasing spark ignition engine efficiency, but their production comes at a substantial economic and environmental cost. The possibility of producing high anti-knock quality gasoline by blending high-octane bio-derived components with low octane naphtha streams is attractive. 2-phenyl ethanol (2-PE), is one such potential candidate that can be derived from lignin, a biomass component made of interconnected aromatic groups. We first ascertained the blending anti-knock quality of 2-PE by studying the effect of spark advancement on knock for various blends 2-PE, toluene, and ethanol with naphtha in a cooperative fuels research engine. The blending octane quality of 2-PE indicated an anti-knock behavior similar or slightly greater than that of toluene, and ethylbenzene, which could be attributed to either chemical kinetics or charge cooling effects. To isolate chemical kinetic effects, a model for 2-PE auto-ignition was developed and validated using ignition delay times measured in a high-pressure shock tube. Simulated ignition delay times of 2-PE were also compared to those of traditional high-octane gasoline blending components to show that the gas phase reactivity of 2-PE is lower than ethanol, and comparable to toluene, and ethylbenzene at RON, and MON relevant conditions. The gas-phase reactivity of 2-PE is largely controlled by its aromatic ring, while the effect of the hydroxyl group is minimal. The higher blending octane quality of 2-PE compared to toluene, and ethylbenzene can be attributed primarily to the effect of the hydroxyl group on increasing heat of vaporization.

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
© King Abdullah University of Science and Technology. All rights reserved