Cyclopentane combustion chemistry. Part I: Mechanism development and computational kinetics

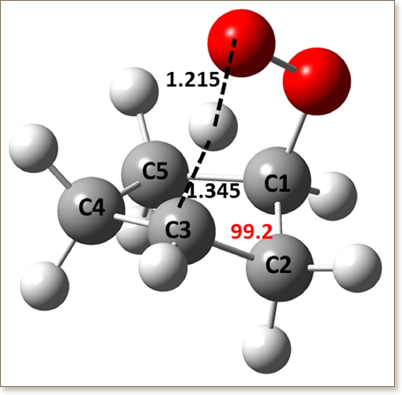
Cycloalkanes are significant constituents of conventional fossil fuels, in which they are one of the main contributors to soot formation, but also significantly influence the ignition characteristics below ∼900 K. This paper discusses the development of a detailed high- and low-temperature oxidation mechanism for cyclopentane, which is an important archetypical cycloalkane. The differences between cyclic and non-cyclic alkane chemistry, and thus the inapplicability of acyclic alkane analogies, required the detailed theoretical investigation of the kinetics of important cyclopentane oxidation reactions as part of the mechanism development. The cyclopentyl + O2 reaction was investigated at the UCCSD(T)-F12a/cc-pVTZ-F12//M06-2X/6-311++G(d,p) level of theory in a time-dependent master equation framework. Comparisons with analogous cyclohexane or non-cyclic alkane reactions are presented. Our study suggests that beyond accurate quantum chemistry the inclusion of pressure dependence and especially that of formally direct kinetics is crucial even at pressures relevant for practical application.
DOI:10.1016/j.combustflame.2017.05.018

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
© King Abdullah University of Science and Technology. All rights reserved