Ignition characteristics of 2-methyltetrahydrofuran: An experimental and kinetic study

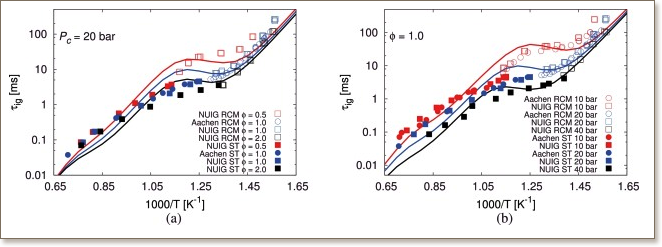
The present paper elucidates oxidation behavior of 2-methyltetrahydrofuran (2-MTHF), a novel second-generation biofuel. New experimental data sets for 2-MTHF including ignition delay time measurements in two different combustion reactors, i.e. rapid
compression machine and high-pressure shock tube, are presented. Measurements for 2-MTHF/oxidizer/diluent mixtures were performed in the temperature range of 639−1413639−1413 K, at pressures of 10, 20, and 40 bar, and at three different
equivalence ratios of 0.5, 1.0, and 2.0. A detailed chemical kinetic model describing both low-and high-temperature chemistry of 2-MTHF was developed and validated against new ignition delay measurements and already existing flame species profiles
and ignition delay measurements. The mechanism provides satisfactory agreement with the experimental data. For identifying key reactions at various combustion conditions and to attain a better understanding of the combustion behavior, reaction
path and sensitivity analyses were performed.

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
© King Abdullah University of Science and Technology. All rights reserved