Integrated In Situ Characterization of a Molten Salt Catalyst Surface: Evidence of Sodium Peroxide and Hydroxyl Radical Formation

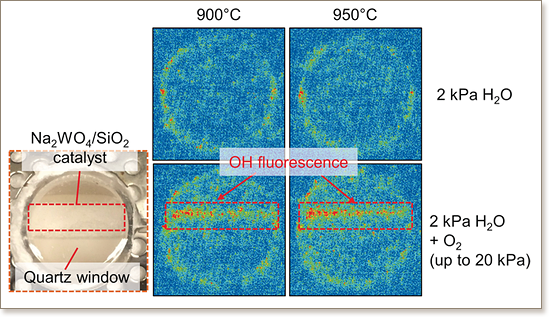
Sodium-based catalysts (such as Na2WO4) were proposed to selectively catalyze OH radical formation from H2O and O2 at high temperatures. This reaction may proceed on molten salt state surfaces owing to the lower melting point of the used Na salts compared to the reaction temperature. This study provides direct evidence of the molten salt state of Na2WO4, which can form OH radicals, using in situ techniques including X-ray diffraction (XRD), scanning transmission electron microscopy (STEM), laser induced fluorescence (LIF) spectrometry, and ambient-pressure X-ray photoelectron spectroscopy (AP-XPS). As a result, Na2O2 species, which were hypothesized to be responsible for the formation of OH radicals, have been identified on the outer surfaces at temperatures of ≥800 °C, and these species are useful for various gas-phase hydrocarbon reactions, including the selective transformation of methane to ethane.
DOI:10.1002/ange.201704758

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
© King Abdullah University of Science and Technology. All rights reserved