Jet-stirred reactor oxidation of alkane-rich FACE gasoline fuels

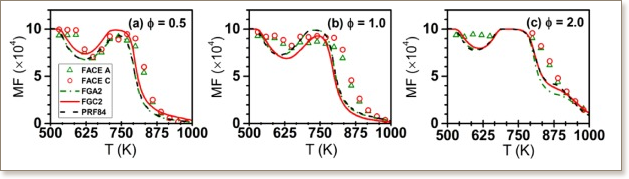
Understanding species evolution upon gasoline fuel oxidation can aid in mitigating harmful emissions and improving combustion efficiency. Experimentally measured speciation profiles are also important targets for surrogate fuel kinetic models. This work presents the low- and high-temperature oxidation of two alkane-rich FACE gasolines (A and C, Fuels for Advanced Combustion Engines) in a jet-stirred reactor at 10 bar and equivalence ratios from 0.5 to 2 by probe sampling combined with gas chromatography and Fourier Transformed Infrared Spectrometry analysis. Detailed speciation profiles as a function of temperature are presented and compared to understand the combustion chemistry of these two real fuels. Simulations were conducted using three surrogates (i.e., FGA2, FGC2, and FRF 84), which have similar physical and chemical properties as the two gasolines. The experimental results reveal that the reactivity and major product distributions of these two alkane-rich FACE fuels are very similar, indicating that they have similar global reactivity despite their different compositions. The simulation results using all the surrogates capture the two-stage oxidation behavior of the two FACE gasolines, but the extent of low temperature reactivity is over-predicted. The simulations were analyzed, with a focus on the n-heptane and n-butane sub-mechanisms, to help direct the future model development and surrogate fuel formulation strategies.

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
© King Abdullah University of Science and Technology. All rights reserved