New insights into methane-oxygen ion chemistry

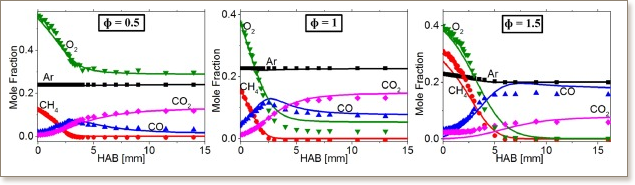
External electric fields may reduce emissions and improve combustion efficiency by active control of combustion processes. In-depth, quantitative understanding of ion chemistry in flames enables predictive models to describe the effect of external electric fields on combustion plasma. This study presents detailed cation profile measurements in low-pressure, burner-stabilized, methane/oxygen/argon flames. A quadrupole molecular beam mass spectrometer (MBMS) coupled to a low-pressure (P = 30 Torr) combustion chamber was utilized to measure ion signals as a function of height above the burner. Lean, stoichiometric and rich flames were examined to evaluate the dependence of ion chemistry on flame stoichiometry. Additionally, for the first time, cataloging of flame cations is performed using a high mass resolution time-of-flight mass spectrometer (TOF-MS) to distinguish ions with the same nominal mass. In the lean and stoichiometric flames, the dominant ions were H3O+, CH3O2+, C2H7O+, C2H3O+ and CH5O+, whereas large signals were measured for H3O+, C3H3+ and C2H3O+ in the rich flame. The spatial distribution of cations was compared with results from numerical simulations constrained by thermocouple-measured flame temperatures. Across all flames, the predicted H3O+ decay rate was noticeably faster than observed experimentally. Sensitivity analysis showed that the mole fraction of H3O+ is most sensitive to the rate of chemi-ionization CH + O ↔ CHO+ + E−. To our knowledge, this work represents the first detailed measurements of positive ions in canonical low-pressure methane flames.

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
© King Abdullah University of Science and Technology. All rights reserved