The influence of n-butanol blending on the ignition delay times of gasoline and its surrogate at high pressures

The influence of n-butanol blending on the ignition delay times of gasoline and its surrogate at high pressures
E. Agbro, A. S. Tomlin, M. Lawes, S. Park, S. M. Sarathy
Fuel 187, pp. 211-219, (2017)
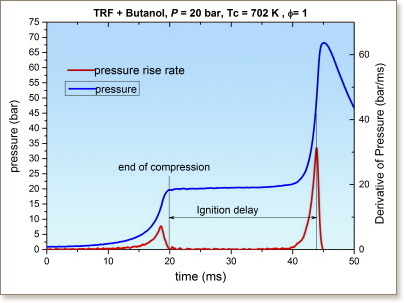
The influence of blending n-butanol at 20% by volume on the ignition delay times for a reference gasoline was studied in a rapid compression machine (RCM) for stoichiometric fuel/air mixtures at 20 bar and 678–858 K. Delay times for the blend lay between those of stoichiometric gasoline and stoichiometric n-butanol across the temperature range studied. At lower temperatures, delays for the blend were however, much closer to those of n-butanol than gasoline despite n-butanol being only 20% of the mixture. Under these conditions n-butanol acted as an octane enhancer over and above what might be expected from a simple linear blending law. The ability of a gasoline surrogate, based on a toluene reference fuel (TRF), to capture the main trends of the gasoline/n-butanol blending behaviour was also tested within the RCM. The 3-component TRF based on a mixture of toluene, n-heptane and iso-octane was able to capture the trends well across the temperature range studied. Simulations of ignition delay times were also performed using a detailed blended n-butanol/TRF mechanism based on the adiabatic core assumption and volume histories from the experimental data. Overall, the model captured the main features of the blending behaviour, although at the lowest temperatures, predicted ignition delays for stoichiometric n-butanol were longer than those observed. A brute-force local sensitivity analysis was performed to evaluate the main chemical processes driving the ignition behaviour of the TRF, n-butanol and blended fuels. The reactions of fuel + OH dominated the sensitivities at lower temperatures, with H abstraction from n-butanol from α and γ sites being key for both the n-butanol and the blend. At higher temperatures the decomposition of H2O2 and reactions of HO2 and that of formaldehyde with OH became critical, in common with the ignition behaviour of other fuels. Remaining uncertainties in the rates of these key reactions are discussed.

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
© King Abdullah University of Science and Technology. All rights reserved