CH4/air homogeneous autoignition: A comparison of two chemical kinetics mechanisms

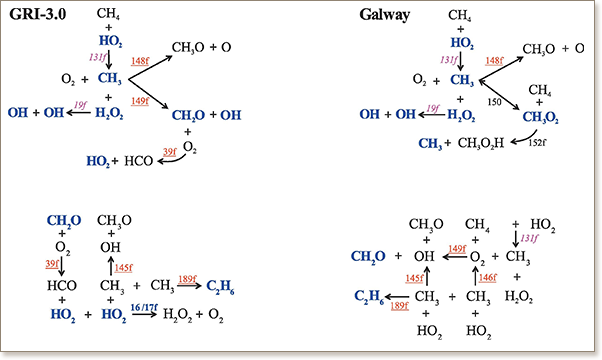
Reactions contributing to the generation of the explosive time scale that characterise autoignition of homogeneous stoichiometric CH4/air mixture are identified using two different chemical kinetics models; the well known GRI-3.0 mechanism (53/325 species/reactions with N-chemistry) and the AramcoMech mechanism from NUI Galway (113/710 species/reactions without N-chemistry; Combustion and Flame 162:315-330, 2015). Although the two mechanisms provide qualitatively similar results (regarding ignition delay and profiles of temperature, of mass fractions and of explosive time scale), the 113/710 mechanism was shown to reproduce the experimental data with higher accuracy than the 53/325 mechanism. The present analysis explores the origin of the improved accuracy provided by the more complex kinetics mechanism. It is shown that the reactions responsible for the generation of the explosive time scale differ significantly. This is reflected to differences in the length of the chemical and thermal runaways and in the set of the most influential species.
DOI: 10.1016/j.fuel.2018.03.025

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
© King Abdullah University of Science and Technology. All rights reserved