Methylcyclohexane pyrolysis and oxidation in a jet-stirred reactor

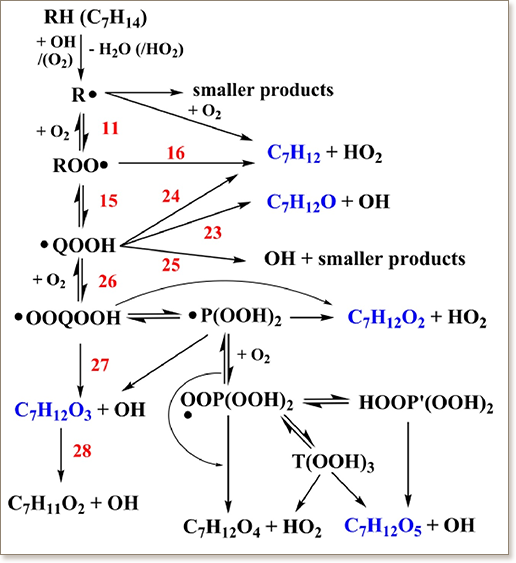
Alkylated cycloalkanes are an important chemical class in conventional fuels. Methylcyclohexane (MCH) is a simple alkylated cycloalkane that is a potential candidate to represent the naphthenic content in transportation fuel surrogates. Detailed experimental speciation data for MCH gas-phase oxidation and pyrolysis is still lacking in the literature. This work investigates the high-temperature pyrolysis and low-temperature oxidation of MCH in a jet-stirred reactor. Under low-temperature conditions, highly oxygenated intermediate species indicative of “alternative isomerization” of hydroperoxyalkylperoxy (OOQOOH) radicals and the subsequent third sequential O2 addition are presented. Furthermore, a detailed chemical kinetic model capable of predicting MCH combustion was developed using new thermodynamic group values and recently published rate coefficients.
Alternative isomerization of OOQOOH radicals and third sequential O2 addition pathways were incorporated into the reaction mechanism. Additionally, an approach for reducing the complexity of the MCH low-temperature chemical pathways has been investigated to limit the arduousness of developing kinetic models. The experimentally measured species concentration profiles at atmospheric pressure, equivalence ratios of 0.25, 1.0, 2, and ∞, and temperatures in the range of 500–1100 K were used to validate and improve the chemical kinetic model. The model was further tested against rapid compression machine ignition delay times taken from literature.
DOI: 10.1016/j.proci.2018.05.086

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
© King Abdullah University of Science and Technology. All rights reserved