Oxidation of 2-methylfuran and 2-methylfuran/n-heptane blends: An experimental and modeling study

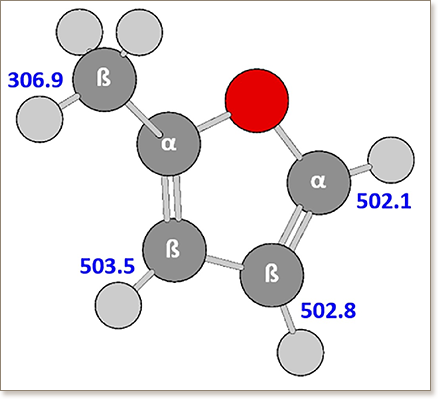
There have been significant advances in understanding ignition behavior of oxygenated biofuels (mainly alcohols) and their blends with conventional fuel components. However, the oxidation behavior of lignocellulosic derived furanic compounds blended with hydrocarbons has received little attention. The present work is an experimental and numerical investigation of 2-methylfuran (2-MF) combustion and its blend with n-heptane. These results are compared with pure n-heptane results to better understand 2-MF reactivity. Ignition delay times of pure 2-MF and the 2-MF/n-heptane (50/50 2-MF/n-heptane molar %) blend in air were measured in three different facilities; a rapid compression machine and two different shock tubes. Experiments were performed in the temperature range of 861–913 K at a pressure of 20 bar for stoichiometric pure 2-MF. The ignition delay times of 2-MF/n-heptane blends were measured in the temperature range of 672–1207 K, at pressures of 10 and 20 bar, and at equivalence ratios of 0.5, 1.0, and 1.5. A comprehensive chemical kinetic model containing low- to high-temperature chemistry of 2-MF and n-heptane was formulated based on a combination of available 2-MF and n-heptane mechanisms and available theoretical studies on 2-MF form literature. The developed detailed kinetic model was validated against the ignition delay data measured in this work as well as against high-temperature shock tube ignition delay, flame speed, and flame species data from literature to ensure the competence of the model. The proposed mechanism predicts the measured and literature data to a reasonable extent. To elucidate fuel specific oxidation pathways, reaction path analyses were performed at various conditions. Furthermore, sensitivity analyses on the ignition delay times were conducted and the dominant reaction pathways in the oxidation of pure and binary mixtures at high, intermediate, and low temperatures were identified. It is found that the competition between n-heptane and 2-MF for ȮH radicals inhibits the consumption of n-heptane and promotes the consumption of 2-MF. This work provides the first insight into the global low-temperature oxidation behavior of a second generation furanic blended with a hydrocarbon.
DOI: 10.1016/j.combustflame.2018.05.032

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
© King Abdullah University of Science and Technology. All rights reserved