Polycyclic aromatic hydrocarbons in pyrolysis of gasoline surrogates (n-heptane/iso-octane/toluene)

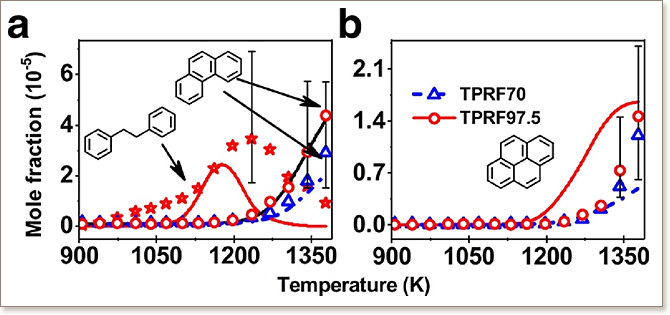
Toluene primary reference fuels (TPRFs), i.e., a ternary mixture of toluene, n-heptane and iso-octane, better match the combustion properties of real gasoline fuels compared to simpler binary n-heptane/iso-octane mixtures. While there has been significant research on combustion of n-heptane/iso-octane mixtures, fundamental data characterizing polycyclic aromatic hydrocarbons(PAHs) formation in TPRFs combustion is lacking, especially under pyrolysis conditions. In this work, the pyrolysis of two TPRF mixtures (TPRF70 and TPRF97.5), representing low octane (research octane number 70) and high octane (research octane number 97.5) gasolines, respectively, was studied in a jet-stirred reactor coupled with gas chromatography (GC) analysis and a flow reactor coupled with synchrotron vacuum ultraviolet photoionization molecular beam mass spectrometry (SVUV-PI-MBMS). The experiments indicate that pyrolysis of TPRF70 produced slightly higher benzene and naphthalene than TPRF97.5. In contrast, TPRF97.5 pyrolysis produced slightly higher phenanthrene and pyrene than TPRF70. The mole fraction profiles of aromatics from benzene to pyrene were used to validate TPRF kinetic models from the literature. Specifically, the KAUST-Aramco PAH Mech 1-GS kinetic model was updated to match and elucidate the experimental observations. The kinetic analysis reveals that propargyl radical is a crucial intermediate forming benzene and naphthalene, while benzyl radical, generated from the dehydrogenation of toluene, plays an important role in formation of larger PAHs.
DOI: 10.1016/j.proci.2018.06.087

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
© King Abdullah University of Science and Technology. All rights reserved