Small ester combustion chemistry: Computational kinetics and experimental study of methyl acetate and ethyl acetate

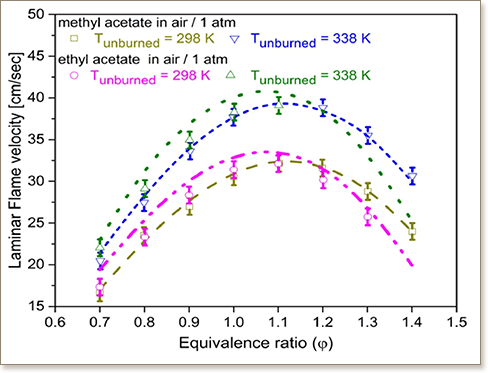
Small esters represent an important class of high octane biofuels for advanced spark ignition engines. They qualify for stringent fuel screening standards and could be synthesized through various pathways. In this work, we performed a detailed investigation of the combustion of two small esters, MA (methyl acetate) and EA (ethyl acetate), including quantum chemistry calculations, experimental studies of combustion characteristics and kinetic model development. The quantum chemistry calculations were performed to obtain rates for H-atom abstraction reactions involved in the oxidation chemistry of these fuels. The series of experiments include: a shock tube study to measure ignition delays at 15 and 30 bar, 1000–1450 K and equivalence ratios of 0.5, 1.0 and 2.0; laminar burning velocity measurements in a heat flux burner over a range of equivalence ratios [0.7–1.4] at atmospheric pressure and temperatures of 298 and 338 K; and speciation measurements during oxidation in a jet-stirred reactor at 800–1100 K for MA and 650–1000 K for EA at equivalence ratios of 0.5, 1.0 and at atmospheric pressure. The developed chemical kinetic mechanism for MA and EA incorporates reaction rates and pathways from recent studies along with rates calculated in this work. The new mechanism shows generally good agreement in predicting experimental data across the broad range of experimental conditions. The experimental data, along with the developed kinetic model, provides a solid groundwork towards improving the understanding the combustion chemistry of smaller esters.
DOI: 10.1016/j.proci.2018.06.178

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
© King Abdullah University of Science and Technology. All rights reserved