
Current Research

Understanding kinetics of chemical reactions occurring in combustion systems requires experimental data on spatial and temporal species profiles. This includes concentrations of reactants, products, stable and reactive intermediate species. Research at the CPC lab endeavors to study the combustion chemistry of gaseous and liquid fuels, through well-defined experiments (0D-1D) and advanced diagnostics (e.g., mass spectrometry and optical).
Jet Stirred Reactors (JSR) are Continuous Stirred Tank Reactors (CSTR) in which the reactants and products are homogenously mixed under well-controlled temperature and pressure conditions. Jets are formed as gas phase reactants flow through nozzles inside the reactor and create turbulent mixing (Fig. 1). This apparatus has been used for studying speciation during gas and liquid fuel pyrolysis and oxidation under various temperatures and pressures relevant to combustion systems. The reactor is kept in an oven that allows for controlling the reactor temperature from room temperature to 1200 K. The system is presently operated at 1 atm with plans to build a pressure chamber to reach up to 10 atm. Probe sampling and non-intrusive diagnostics are adopted at CPC lab to elucidate the formation of stable products and reactive intermediates, which are valuable to explore the reaction mechanism and develop detailed kinetic models of fuels and their surrogates.

Figure 1: Picture of JSR at KAUST
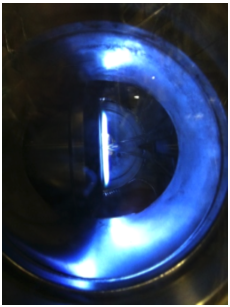
Ions in flames have been studied for decades in an effort to understand the underlying chemistry. Recent studies indicate the use of external electric fields on engine combustion can reduce emissions and increase efficiency. In our laboratory, we study
ions in low-pressure premixed flames, as these permit high spatial resolution. In this research, we identified and quantified ions in low-pressure flames, providing experiment data to help with developing ion chemistry mechanisms.
The scheme
of experiment set up is in Figure 3. The MBMS system is coupled to a combustion chamber capable of sustaining flames at low pressures and a quadrupole mass spectrometer is used to separate the products. A chiller was applied on the McKenna burner
to establish premixed flat flames at low pressure with Argon as the diluent gas. The McKenna burner was mounted on a motorized translation stage. Ions in the centerline of the flame were sampled by a detector, and the signals were then transformed
to mole fraction by using mass discrimination calibration and FKT calibration. Flame temperature was measured using a thermocouple. Neutrals were also measured by electron ionization in MBMS to validate the flame structure.
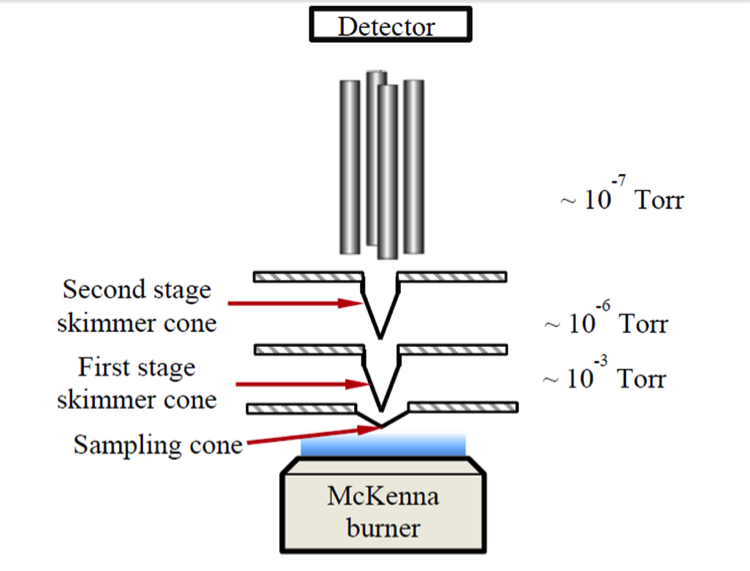
Figure 3. Scheme of low pressure flame coupled with a quadruple MBMS
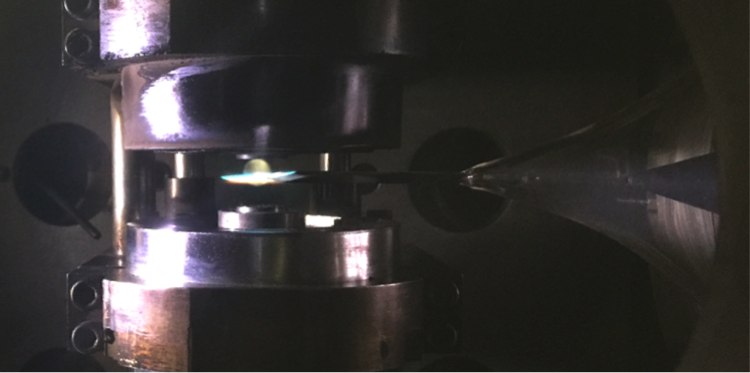
A better knowledge of high-temperature combustion processes in transport affected environments would facilitate the understanding of ignition events in practical combustors. Our counterflow facility is an atmospheric pressure setup designed to understand the interaction between flow and chemistry in diffusion flames. Our main focus is in measurements of autoignition temperature, extinction limits, and flame structure of various liquid and gaseous fuels. The experimental data generated is used to validate high temperature kinetics and transport in reacting flows. The current fuels under study include blends of natural gas mixtures with DME and H2, light & heavy naphtha and gasoline fuels.

Soot formation within combustion applications has substantial malignant impact on the environment and humans. The formation of soot from fuels combustion is a complicated process, starting with the formation of the PAHs molecule precursors, followed by
first ring formation, PAHs growth, Particle inception, particle growth, and oxidation.
In this research we investigate the effect of molecular fuel structure, dopants addition, and the characteristic flow time on the formation of PAHs
and their precursors in a counterflow diffusion flame burner. This research is being conducted in the Advanced Light Source at Lawrence Berkeley National Laboratory using synchrotron vacuum-ultraviolet molecular beam mass spectrometry.
In combustion and pyrolysis systems, species produced by chemical reactions should be analyzed with the appropriate analytical device. The most common analytical technique which is extensively used for the identification and the quantification of species
in gas-phase is Gas Chromatography (GC). This system is well adapted to the analysis of most stable molecules such as permanent gases and hydrocarbons. However, this doesn’t include unstable molecules and radical intermediates species.
At the CPC lab, the GC employs an advanced electronic pneumatic control (EPC) modules and a high performance GC oven temperature control. These features are keys of the GC’s superior performance in all applications needed. This system is also
equipped with three different detectors (one FID and two TCD) allowing the detection of several products depending on their properties and on the calibration. Moreover, this kind of GC may be combined with mass spectrometry to improve identification
of measured species.
Laser diagnostics are widely used to study combustion kinetics, due to its high resolution and high accuracy. In the CPC lab, we use a Ti:S laser absorption technique that provides non-intrusive measurements of radicals in JSR oxidation. Experimental
data are used for validating combustion kinetic mechanisms.
A schematic of the experiment setup is shown in Figure 2. A pump laser at 532 nm is transformed into 760-1000 nm laser light by a Ti:S crystal, and then an optical grid is used
to select the desired wavelength. The light can be doubled and/or quadrupled later by a second and/or fourth harmonic generator box. The light is separated into two beams, one as reference beam and another one as signal beam. A silicon detector detects
both beams and the data is collected with an oscilloscope. The absorbance was determined by taking ratio of two signals, and by knowing absorption cross-section, the species mole fraction can be calculated using the Beer-Lambert Law.
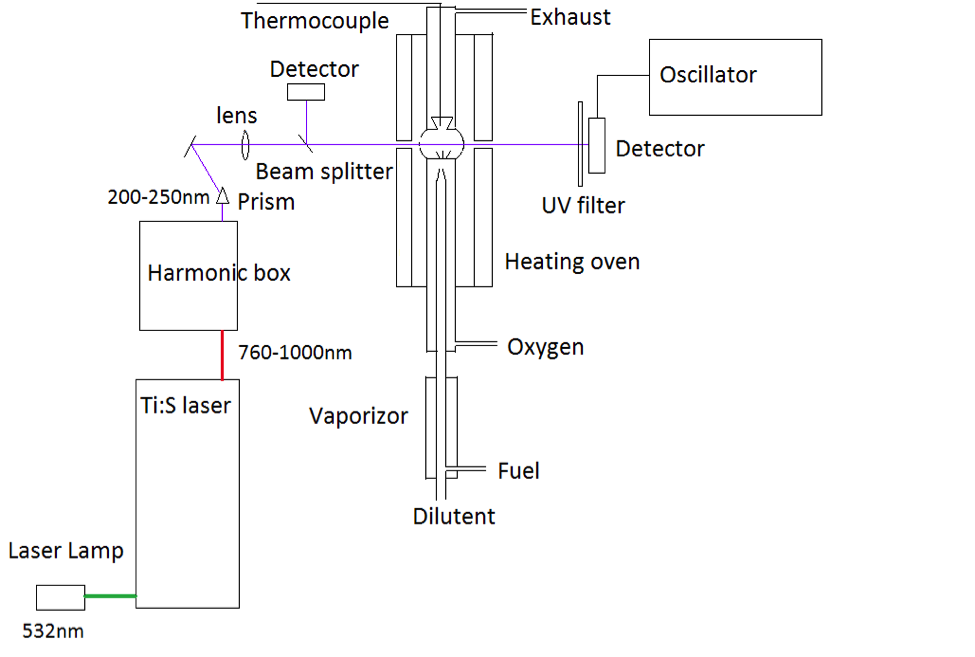
Figure 2. Scheme of laser diagnostic for JSR oxidation
In addition, our research is facilitated by the various diagnostics in the core labs at KAUST, like GC-FID/TCD/MS, APCI/ESI-orbitrap mass spectrometry, and NMR, etc.

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
© King Abdullah University of Science and Technology. All rights reserved